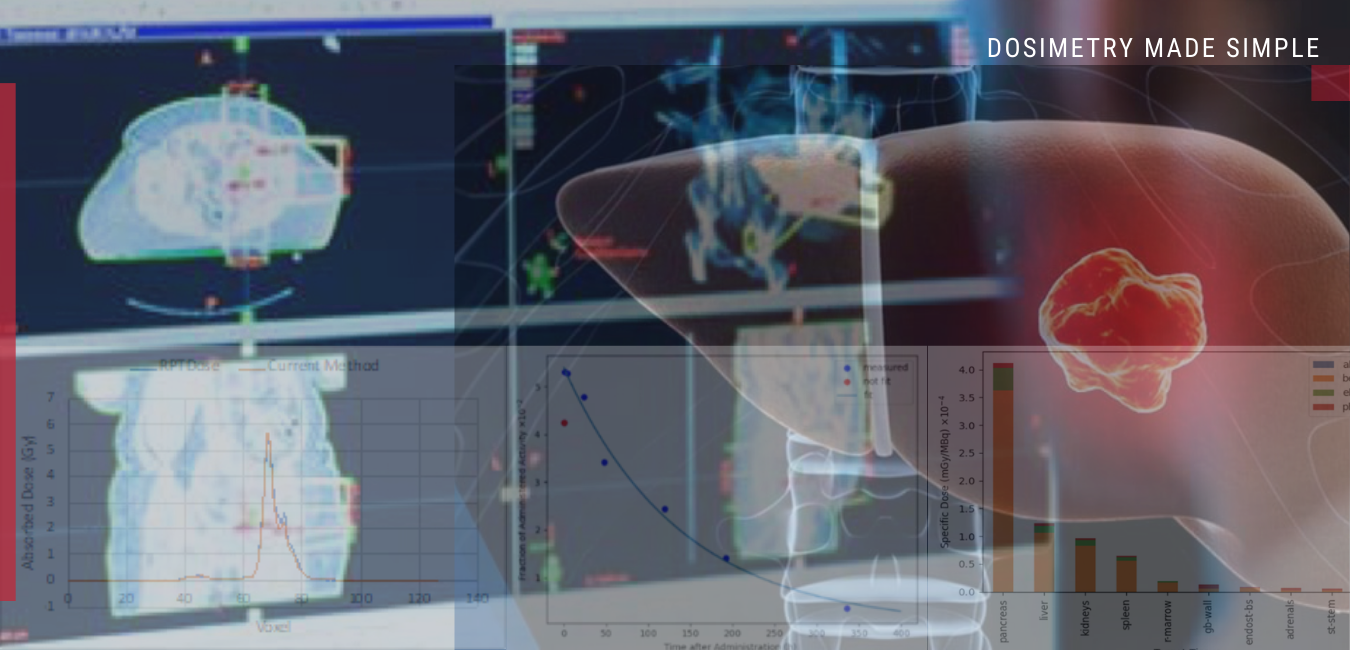
Rapid’s 3D-RD-S Secures UK Market Access Under CE Mark
Baltimore, Maryland, – January 14 , 2026 — Radiopharmaceutical Imaging and Dosimetry, LLC (Rapid®) today announced that the UK Medicines and Healthcare products Regulatory Agency (MHRA) has acknowledged the registration of…